Radium
88
Ra
Kumpulan
2
Kala
7
Blok
s
Proton
Elektron
Neutron
88
88
138
Ciri-Ciri Am
Nombor atom
88
Berat atom
[226]
Nombor jisim
226
Kategori
Logam alkali bumi
Warna
Perak
Radioaktif
Ya
From the Latin word radius meaning ray
Struktur hablur
Pusat Badan Kubik
Sejarah
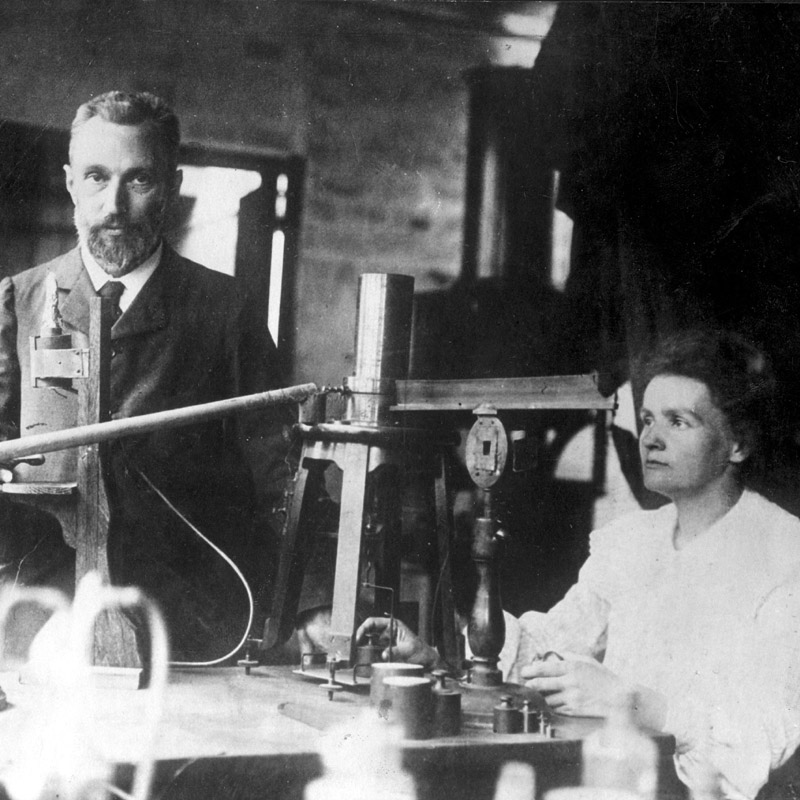
Radium was discovered by Marie Curie and Pierre Curie in 1898.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
Bilangan elektron per petala
2, 8, 18, 32, 18, 8, 2
Konfigurasi elektron
[Rn] 7s2
Radium imparts a carmine red color to a flame
Ciri-Ciri Fizikal
Fasa
Pepejal
Ketumpatan
5.5 g/cm3
Takat lebur
973.15 K | 700 °C | 1292 °F
Takat didih
2010.15 K | 1737 °C | 3158.6 °F
Haba pelakuran
8 kJ/mol
Haba pengewapan
125 kJ/mol
Muatan haba molar
- J/g·K
Banyak pada kerak bumi
9.9×10-12%
Banyak pada alam semesta
Tiada
Nombor CAS
7440-14-4
Nombor PubChem CID
6328144
Ciri-Ciri Atom
Jejari atom
-
Jejari kovalen
221 pm
Keelektronegatifan
0.9 (Skala Pauling)
Kebolehan mengion
5.2784 eV
Isipadu atom
45.20 cm3/mol
Daya pengaliran terma
0.186 W/cm·K
Keadaan pengoksidaan
2
Aplikasi
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium is highly radioactive and carcinogenic
Isotop
Isotop stabil
-Isotop tidak stabil
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra