Aktinium
89
Ac
Kumpulan
Tiada
Kala
7
Blok
f
Proton
Elektron
Neutron
89
89
138
Ciri-Ciri Am
Nombor atom
89
Berat atom
[227]
Nombor jisim
227
Kategori
Aktinid
Warna
Perak
Radioaktif
Ya
From the Greek aktis, aktinos, meaning beam or ray
Struktur hablur
Pusat Wajah Kubik
Sejarah
André-Louis Debierne, a French chemist, discovered actinium in 1899.
He separated it from pitchblende residues left by Marie and Pierre Curie after they had extracted radium.
Friedrich Oskar Giesel independently discovered actinium in 1902 as a substance being similar to lanthanum.
He separated it from pitchblende residues left by Marie and Pierre Curie after they had extracted radium.
Friedrich Oskar Giesel independently discovered actinium in 1902 as a substance being similar to lanthanum.
Bilangan elektron per petala
2, 8, 18, 32, 18, 9, 2
Konfigurasi elektron
[Rn] 6d1 7s2
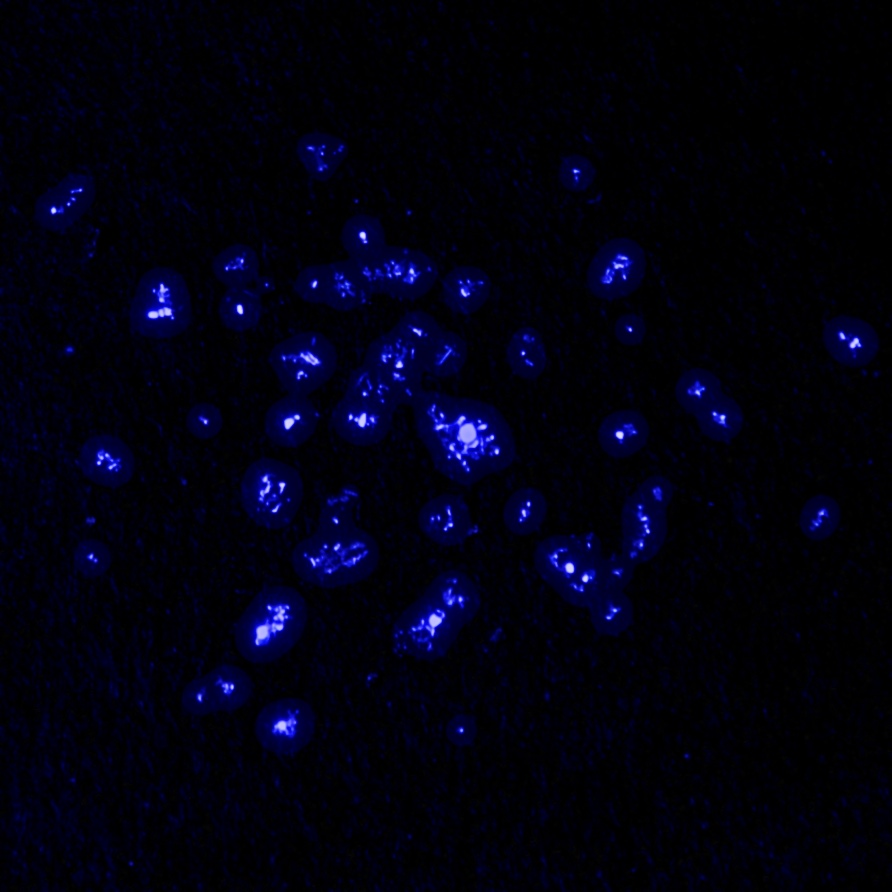
Actinium glows in the dark with a pale blue light
Ciri-Ciri Fizikal
Fasa
Pepejal
Ketumpatan
10.07 g/cm3
Takat lebur
1323.15 K | 1050 °C | 1922 °F
Takat didih
3471.15 K | 3198 °C | 5788.4 °F
Haba pelakuran
14 kJ/mol
Haba pengewapan
400 kJ/mol
Muatan haba molar
0.12 J/g·K
Banyak pada kerak bumi
Tiada
Banyak pada alam semesta
Tiada
Nombor CAS
7440-34-8
Nombor PubChem CID
Tiada
Ciri-Ciri Atom
Jejari atom
-
Jejari kovalen
215 pm
Keelektronegatifan
1.1 (Skala Pauling)
Kebolehan mengion
5.17 eV
Isipadu atom
22.54 cm3/mol
Daya pengaliran terma
0.12 W/cm·K
Keadaan pengoksidaan
3
Aplikasi
Actinium is used as an active element of radioisotope thermoelectric generators, for example in spacecraft.
The medium half-life of 227Ac makes it very convenient radioactive isotope in modeling the slow vertical mixing of oceanic waters.
225Ac is applied in medicine to produce 213Bi in a reusable generator or can be used alone as an agent for radiation therapy.
The medium half-life of 227Ac makes it very convenient radioactive isotope in modeling the slow vertical mixing of oceanic waters.
225Ac is applied in medicine to produce 213Bi in a reusable generator or can be used alone as an agent for radiation therapy.
Actinium is highly radioactive
Isotop
Isotop stabil
-Isotop tidak stabil
206Ac, 207Ac, 208Ac, 209Ac, 210Ac, 211Ac, 212Ac, 213Ac, 214Ac, 215Ac, 216Ac, 217Ac, 218Ac, 219Ac, 220Ac, 221Ac, 222Ac, 223Ac, 224Ac, 225Ac, 226Ac, 227Ac, 228Ac, 229Ac, 230Ac, 231Ac, 232Ac, 233Ac, 234Ac, 235Ac, 236Ac